1. What is medical equipment?
Medical equipment includes tools, devices, materials, and chemicals used for in vitro diagnosis, utilized according to the owner's instructions for purposes such as:
-
Diagnosing, preventing, monitoring, treating, and alleviating diseases or injuries.
-
Examining, replacing, adjusting, or supporting anatomical and physiological processes.
-
Supporting life maintenance.
-
Controlling pregnancy.
-
Sterilizing medical equipment (excluding chemicals, pesticides, and disinfectants used indoors).
-
Using for medical equipment.
The process of importing medical equipment is divided into four main areas:
-
Classification of medical equipment (A, B, C, D).
-
Procedures for registering the free circulation of medical equipment.
-
Procedures for obtaining an import license for medical equipment (applicable to types B, C, D).
-
Commercial procedures for medical equipment.
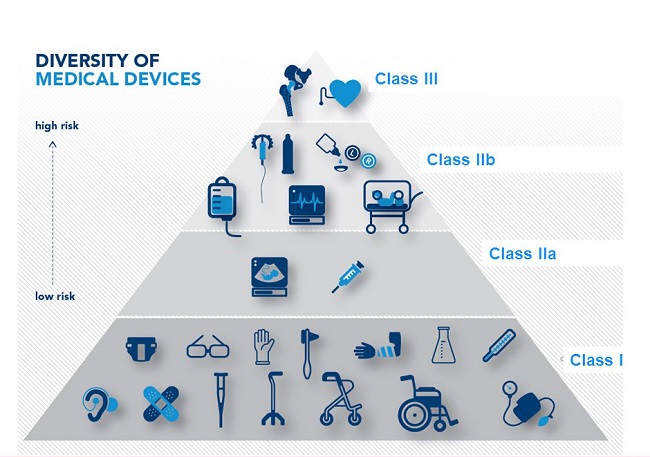
2. Classification of medical equipment
2.1. Classification of medical equipment
When trading medical equipment, it is necessary to check compatibility with Article 4 of Decree 36/2016 and Circular 39/2016/TT-BYT to determine the type of equipment (A, B, C, D).
-
Type A: Medical equipment classified by group.
-
Types B, C, D: Require an import license if listed in the product categories as per Circular 30/2015.
2.2. Procedure for classifying medical equipment
The healthcare sector is strictly managed by the Ministry of Health. The Department of Medical Equipment and Health Works (DMEHW) has the authority to import medical equipment. Steps to follow:
-
Prepare the documents including:
-
Application for classification.
-
Technical documentation describing functions and specifications.
-
Quality management certification.
-
Classification and free circulation certificate (if available).
-
-
Submit the application to the Department of Medical Equipment and Health Works.
-
Monitor feedback and supplement if necessary.
-
Receive classification results.
3. Procedures for registering the circulation of medical equipment
To circulate foreign medical equipment, it is necessary to follow the registration procedure as stipulated in Decree 36/2016/NĐ-CP. Documents include:
-
Registration application.
-
Classification of medical equipment.
-
Quality management certification.
-
Power of attorney from the owner.
-
Technical description of the equipment. For types C and D medical equipment, additional testing information and conformity certification are required.
4. Procedures for obtaining an import license for medical equipment
Medical equipment requires an import license from the Ministry of Health, as regulated by Circular 30/2015/TT-BYT.
4.1. Application for an import license
The application submitted to the Department of Medical Equipment and Health Works includes:
-
Application for licensing.
-
Business registration certificate.
-
Quality management certification.
-
Product description and technical documentation. If the application is valid, the license will be issued within 15 days.
4.2. Procedure for applying for an import license
The procedure includes:
-
Applying for a license at the Ministry of Health.
-
Waiting for feedback.
-
Supplementing documents if necessary.
-
Receiving results.
5. Procedures for importing medical equipment
Steps to follow:
-
Apply for an import license (as stated in section 4).
-
Submit documents to the customs authority.
-
Carry out customs procedures, including documents such as business invoices and product classification records.












